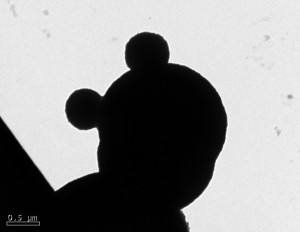Colorado College
Chemistry
FBRI Project: Conversion of Furfural from Agro-Industrial Residue into Higher-Value Added Biomaterial for Water Purification
Without water, life cannot exist. Water quality is a serious issue around the world, and nearly 2 billion people lack access to reliable clean water sources. Water pollution can be natural, such as bacteria and viruses, but man-made pollutants such as dyes, pharmaceuticals, and industrial byproducts are of importance as well. Traditional water purification techniques are oftentimes unable to remove these man-made pollutants, and the world demand for clean water is too great for conventional systems to support alone. New techniques for purifying water are needed that are cheap, environmentally sustainable, and more effective against man-made pollutants. A promising area of research in water purification technology is a group of techniques known as Advanced Oxidative Processes (AOP’s). AOP’s vary widely, but they all make use of chemistry to generate extremely reactive molecules called hydroxyl radicals, which can destroy pollutants more efficiently and more completely than traditional water purification techniques like chlorination. Many AOP’s are expensive, toxic, and unsustainable, so they are not used on a large scale. However, one AOP, known asphotocatalysis, uses safe, reusable catalysts and solar energy to generate hydroxyl radicals to destroy pollutants. This project is to investigate how to make these catalysts, nanoparticle spheres made from TiO2, C, and Cu, by using a waste product from the forest bio-products industry, furfural, and advanced chemistry techniques. The nanoparticle catalysts will be synthesized, and then characterized by electron microscopes (transmission electron microscopy; scanning electron microscopy), by analyzing the way x-rays scatter off of them (x-ray diffraction spectroscopy; energy-
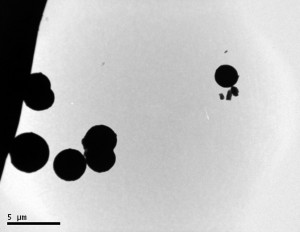
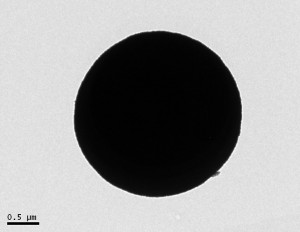
Below are pictures of nanospheres that Jarod synthesized from the transmission electron microscope. The scale bar next to the images gives you an idea about the size. They average around 1-2 µm.